Peptide Diversification
Methods for the chemoselective modification of amino acids and peptides are powerful techniques in biomolecular chemistry. Non-natural peptides are of key relevance for numerous applied research areas, ranging from proteomics and diagnosis to asymmetric synthesis and drug delivery. In this context, C–H activation chemistry is a powerful step- and atom-economical approach for the late-stage modification of peptides.
Selected publications:
A) A. Kopp, T. Oyama, L. Ackermann, "Fluorescent Coumarin-Alkynes for Labeling of Amino Acids and Peptides via Manganese(I)-Catalyzed C–H Alkenylation", Chem. Commun. 2024, 60, 5423-5426. Abstract
B) J. Struwe, T. Oyama, F. Gallou, L. Ackermann, "Manganese(I)-Catalyzed C–H Allylation of Tryptophanes and their Oligopeptides on Water", Synlett 2024, 35, A-E. Abstract (Special issue dedicated to Prof. Keith Fagnou )
C) M. A. Brimble, L. Ackermann, Y.-M. Li , M. Raj, "Chemoselective Methods for Labeling and Modification of Peptides and Proteins" (Editorial) Org. Lett. 2023, 25, 6605–6606. Abstract
D) T. Oyama, L. Mendive-Tapia, V. Cowell, A. Kopp, M. Vendrell, L. Ackermann, "Late-Stage Peptide Labeling with Near-Infrared Fluorogenic Nitrobenzodiazoles by Manganese-Catalyzed C–H Activation" Chem. Sci. 2023, 14, 5728-5733. Abstract
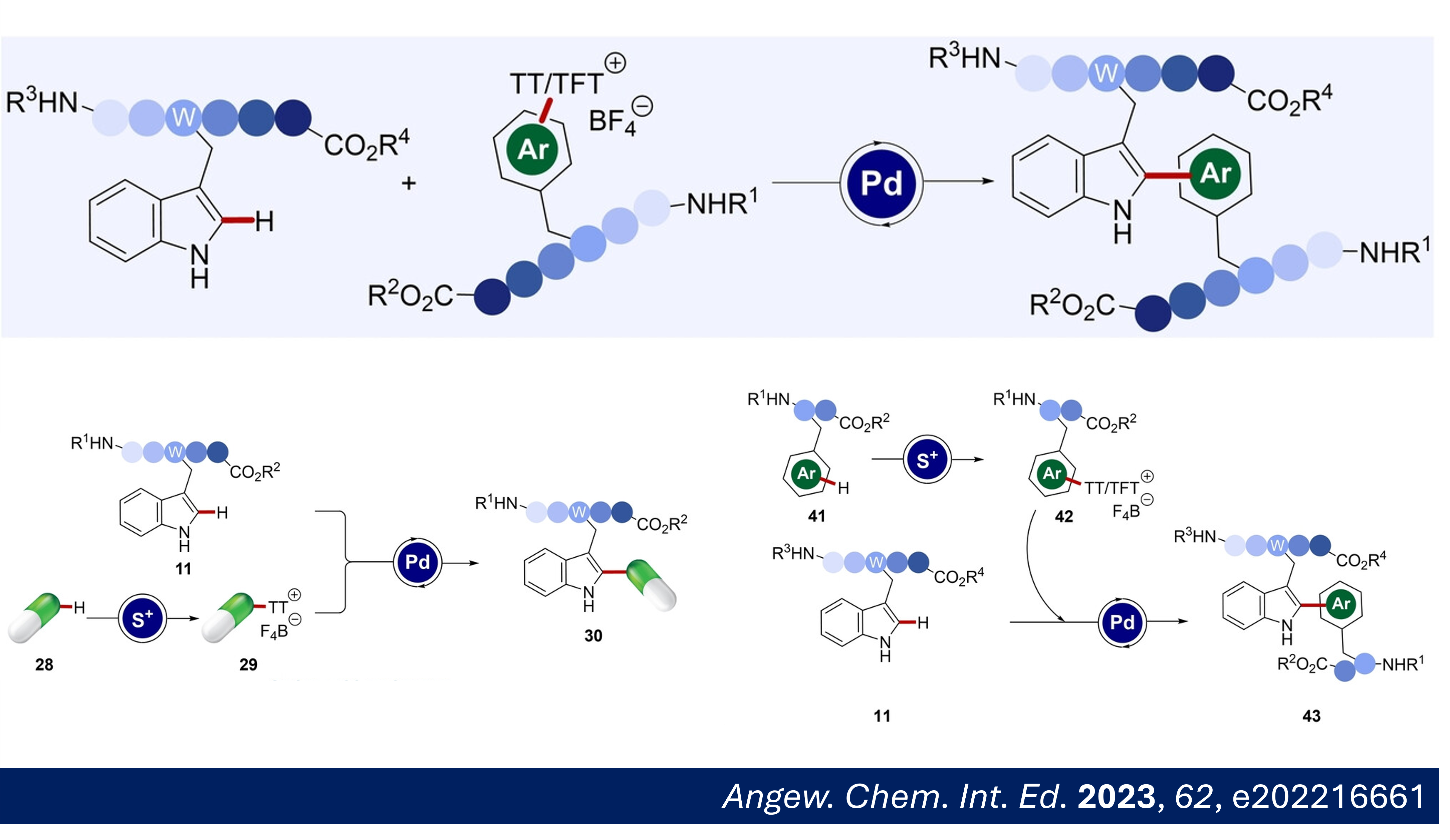
E) N. Kaplaneris, A. Puet, F. Kallert, J. Pöhlmann, L. Ackermann, "Late-stage C–H Functionalization of Tryptophan-containing Peptides with Thianthrenium Salts: Conjugation and Ligation" Angew. Chem. Int. Ed. 2023, 62, e202216661. Abstract
