Remote Functionalization
The control of regioselectivty in C–H activations is usually restricted to entropically-favored intramolecular reactions, functionalizations of electronically-biased heteroarenes, or proximity-induced, ortho-C–H functionalizations. In sharp contrast, our group has accomplished a wide range of remote C–H functionalizations by ruthenium catalysis.
Selected publications:
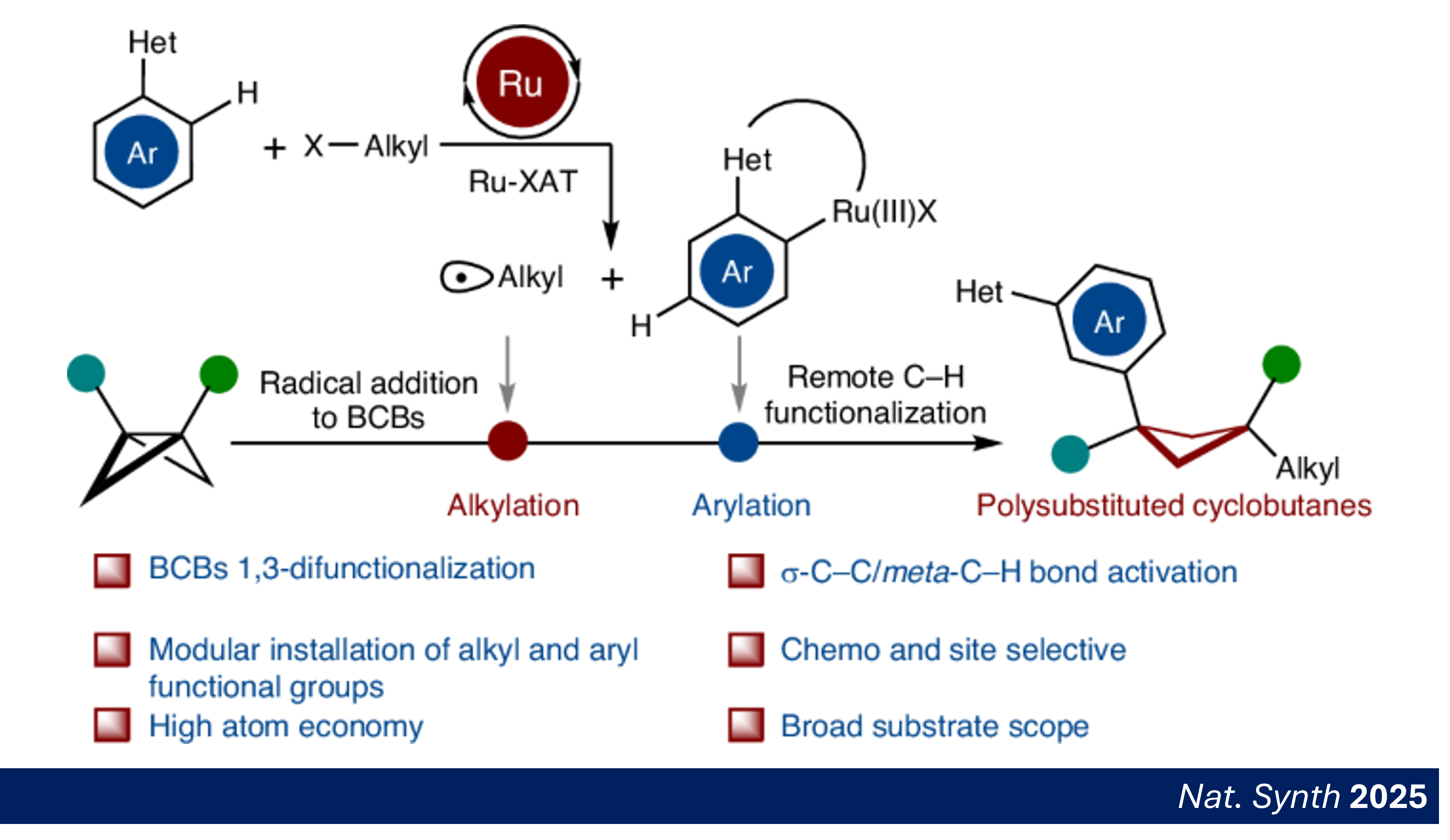
A) S. Chen, Z. Xu, B. Yuan, X.-Y. Gou, L. Ackermann, "Difunctionalization of bicyclo[1.1.0]butanes enabled by merging C–C cleavage and ruthenium-catalysed remote C–H activation", Nat. Synth. 2025, in press. Abstract
B) H. Lan, Y. Liu, L. Ackermann, L. Wang, D. Wang, "Ruthenium(II)-Catalyzed Remote C–H Alkylation of Arenes Using Diverse N-Directing Groups through Aziridine Ring Opening", Org. Lett. 2024, 26, 7993-7998. Abstract
C) S. Chen, B. Yuan, Y. Wang, L. Ackermann, "Ruthenium-Catalyzed Remote-Difunctionalization of Nonactivated Alkenes for Double meta-C(sp2)−H/C-6(sp3)−H Functionalization" Angew. Chem. Int. Ed. 2023, 62, e202301168. Abstract
D) D. Wang, B. Yuan, J. Xu, L. Ackermann, "Electrochemical Rearrangement for Remote Functionalizations of Unactivated Alkenes" Chem. Eur. J. 2023, 28, e202300600. Abstract
E) J. Wu, N. Kaplaneris, J. Pöhlmann, T. Michiyuki, B. Yuan, L. Ackermann, "Remote C–H Glycosylation by Ruthenium(II) Catalysis: Modular Assembly of meta-C-Aryl Glycosides" Angew. Chem. Int. Ed. 2022, 61, e202208620. Abstract
